What is an ISO Class 7 Cleanroom?
What is a Cleanroom classification?
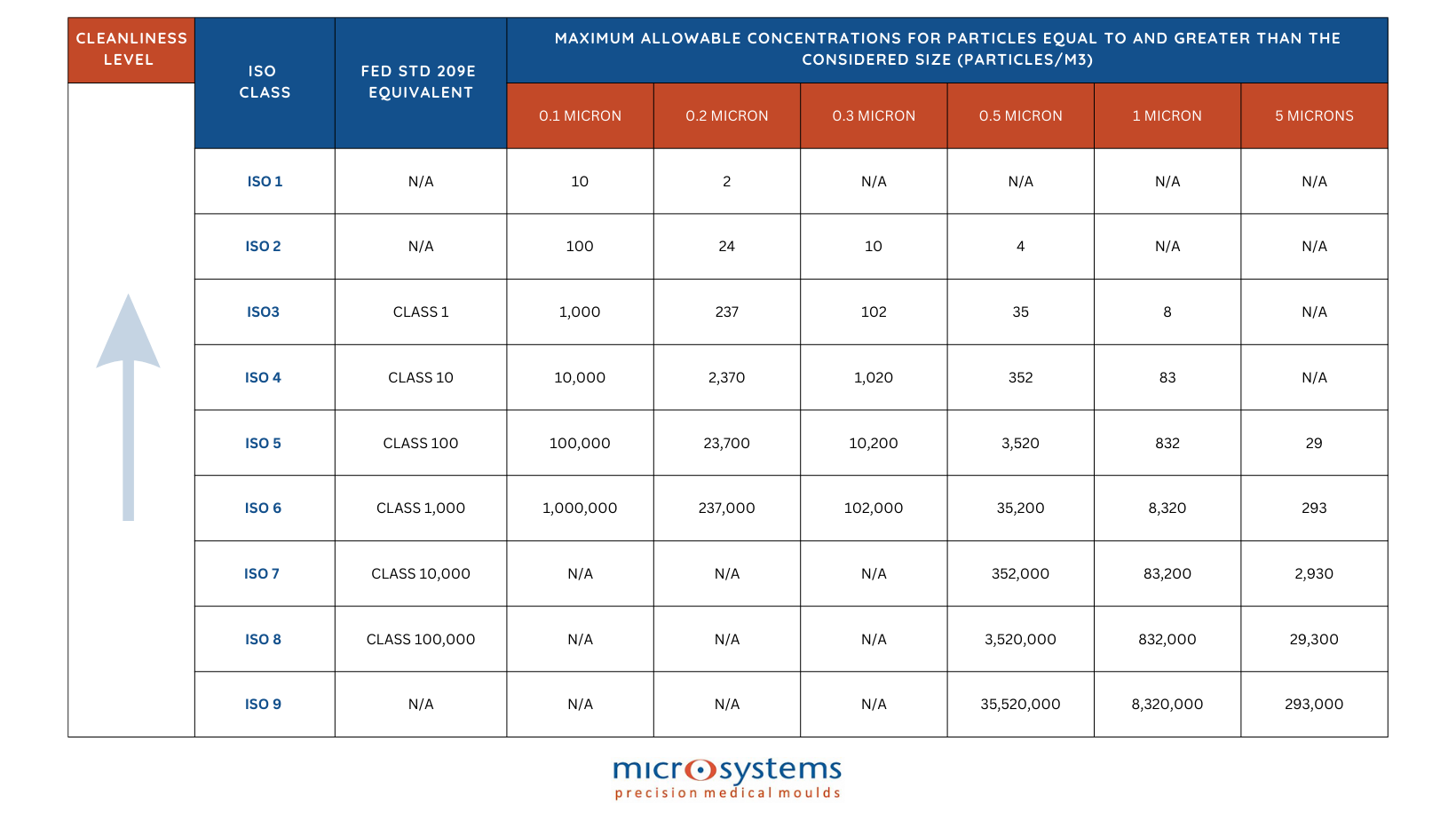
Cleanrooms are categorised based on the quantity and size of particles allowed per air volume. The ISO classifies particles into 9 groups, where 1 is the least stringent and allows the highest concentration of particles per cubic metre or metre of air.
Production of consumable, medicinal, and other items requiring hygienic conditions typically requires a cleanroom. They are made to allow for the tight management and monitoring of particle flow and pollutants in order to meet specifications.
What is an ISO Class 7 Cleanroom?
ISO 7 is one of the most popular cleanroom classifications. An ISO Class 7 Cleanroom would have at least 60 air changes per hour and less than 352,000 particles larger than 0.05 microns per cubic metre, and less than 2,930 particles per cubic metre greater or equal to 5 microns. In comparison, there would be almost 100 times as many particles per metre in a conditioned office environment (a non-cleanroom).
The particle counts for each cubic metre of air must remain below allowed limits in order to retain ISO Class 7 cleanroom accreditation:
- ≥ 0.5 µm particles, maximum count is 352,000
- ≥ 1.0 µm particles, maximum count is 83,200
- ≥ 5.0 µm particles, maximum count is 2,930
Biotechnology, pharmaceuticals, nanotechnology, compounding, and a host of other cleantech production applications—including ion lithium batteries, solar energy, medical devices, film/packaging, nutraceuticals, and some food packages—all make use of ISO 7 cleanrooms.
Overall, the requirements for an ISO Class 7 cleanroom are:
- 60 to 90 air changes per hour
- ≥ 0.5 µm particles, maximum count is 352,000, ≥ 1.0 µm particles, maximum count is 83,200, ≥ 5.0 µm particles, maximum count is 2,930
- ISO 14644-2 testing every 6 months
- HEPA filters (99.9997% efficient at 0.3 microns)
- Sticky mats at entry/exit points
- A separate area for gowning
What are the design specifications of ISO Class 7 Cleanroom?
Airflow and Air Change Rates per hour (ACPH):
- Mixed or non-unidirectional air flow patterns are usually adopted. Most frequently, airflow is vertical and flows from the ceiling to the floor, with low wall returns serving as the means of conditioning and filtering the air.
- Normal variations in air: 60–90 air changes in a single hour.
- A randomised array with filtering situated over the highest control sections provides ceiling coverage of 15-20%.
Filtration and Particle Control:
- Measurement with focus on particles larger than 0.5µ and less than 5.0µ.
- High Efficiency Penetration Air Filtration (HEPA): Effectiveness of filtering At 0.3 microns, 99.99% final filtering, which depends on the application, is usually carried out by terminal HEPA modules or HEPA FFU (fan filter modules) at the cleanroom’s air entrance point.
- Particles per cubic metre (PPC): 352,000 at 0.5µ.
- Environmental factors including illumination, temperature, humidity, noise thresholds, outgassing, static control, and lighting intensity are frequently determined by the demands of the particular process carried out in the cleanroom.
Architectural Finishes:
- Air returns from low walls.
- Wall systems: based on the cleanroom’s intended use, modular wall systems are constructed. In order to meet sterile criteria, biotech applications need non-shedding systems with monolithic seams and coving to eliminate 90° angles, allowing for wet washing or VHP fogging. Non-shedding systems are used in nanotechnology and other non-sterile cleanroom types, and they are routinely vacuumed or dry wiped.
- Ceiling systems: Depending on how the cleanroom is being used, specially designed ceiling systems are used for ISO 7 requirements. In order to retain monolithic washable surfaces and access to maintain lights and filter media from the top side of the ceiling without going beyond the certified and validated space, biotech and pharmaceutical applications require walkable or panelised ceiling systems. When equipment like lights or filters are not in use, gasketed grid systems with non-shedding blank ceiling tiles are frequently used in non-sterile applications like nanotechnology.
- Depending on the application, flooring systems with certain qualities (such as chemical resistance, coving, static control, rolling weight loading / impact resistance, non-slip) are most frequently resinous floor systems or heat sealed vinyl flooring systems.
- Other architectural characteristics, such as different kinds of doors, glass walls, pass-throughs, viewing windows, sprinkler heads, fire suppression, network and Wi-Fi connectivity, security cameras, and access control devices.
Gowning room:
- In addition to making gowning operations easier to carry out, designated gowning rooms lessen the possibility of operator-born contamination entering the clean room through hands, feet, clothes, or equipment.
- A gowning room with ISO class 8 air quality is typically requested by ISO class 7 cleanrooms, however this isn’t always the case.
- The USP chapters 797 and 800 standards provide the cleanroom criteria for ante-rooms, buffer rooms and principal engineering controls (PECs) in facilities that fall within its purview. The air quality in buffer rooms ought to be at least ISO Class 7. Positive pressure buffer room access ante-rooms must be at least ISO Class 8 classified.
Sanitation guidelines:
- Always clean from top to bottom and from cleanest to dirtiest.
- Select single-use, non-shedding, non-linting cleanroom wipes.
- Instead of using circular movements, use unidirectional, slightly overlapping strokes.
- Swap out used wipes often.
- Buckets, mops, and cleaning supplies should be kept separate for every room or space.
- It is important to check the product label for the correct contact time as disinfectant dwell time.

What is the ISO 14644-2 test of ISO Class 7 Cleanroom?
- Particle Count Test: 12 months is the maximum time interval allowed under ISO 14644-1 Annex A.
- Air Pressure Cascade: 12 months is the maximum duration allowed under ISO 14644-1 Annex B5.
- Airflow: ISO 14644-1 Annex B4 test protocol, maximum time interval of 12 months
Although the aforementioned requirements can be verified and tested every 12 months, it is advised that this be done on a six-month timetable.
Why choose medical device manufacturers with ISO Cleanrooms?
As the healthcare sector is renowned for its strict laws and high standards, which guarantee the security of its delicate products,medical device manufacturers need to comply with all applicable laws and regulations on a global scale.

By choosing medical device and part manufacturers with the appropriate ISO Cleanrooms, OEMs could ensure their suppliers meet their high standards for the end products, protecting the end-users, securing medical device certifications and guaranteeing reliability in their production chain. Medical device manufacturers with ISO Cleanrooms like Micro Systems offer their customers a high degree of operational and functional control, and advantages of efficiency, competency, accountability, and ability to adhere to sterility and global regulatory norms. At Micro Systems, a complete process from mold design through micro mold manufacturing and development to validation is under ISO 9001 and ISO 13485 standards, and backed up by an extensively equipped Metrology department to ensure the highest quality and assurance.
Contact us today to discuss your Mold tooling and Injection Molding projects!





