What are ISO Cleanroom Classifications for Medical Devices?
Products in many industries like life sciences (medical devices, healthcare, food, pharmaceuticals), aerospace microelectronics, optics, nuclear and many more usually require a highly hygienic production environment. Manufacturers in those industries adopt different ISO Cleanroom classifications, depending on each product’s requirements, to ensure their products meet the highest standards required.

What is ISO Cleanroom?
According to ISO 14644, cleanrooms and associated controlled environments are designed to provide for the control of contamination to levels appropriate for accomplishing contamination-sensitive activities. In particular, a cleanroom is defined as a room that is created, built, and used in a way to manage the entry, creation, and retention of particles inside the room, as well as the concentration of airborne particles within the room.
What are ISO Cleanroom Classifications?
Within the ISO Cleanroom, there are 9 classifications, ranking from ISO 1 to ISO 9. The technique assigns a score based on the size and amount of airborne particles per m3, with 1 being the cleanest cleanroom environment. Simply put, the air in the room is cleaner the lower the number.
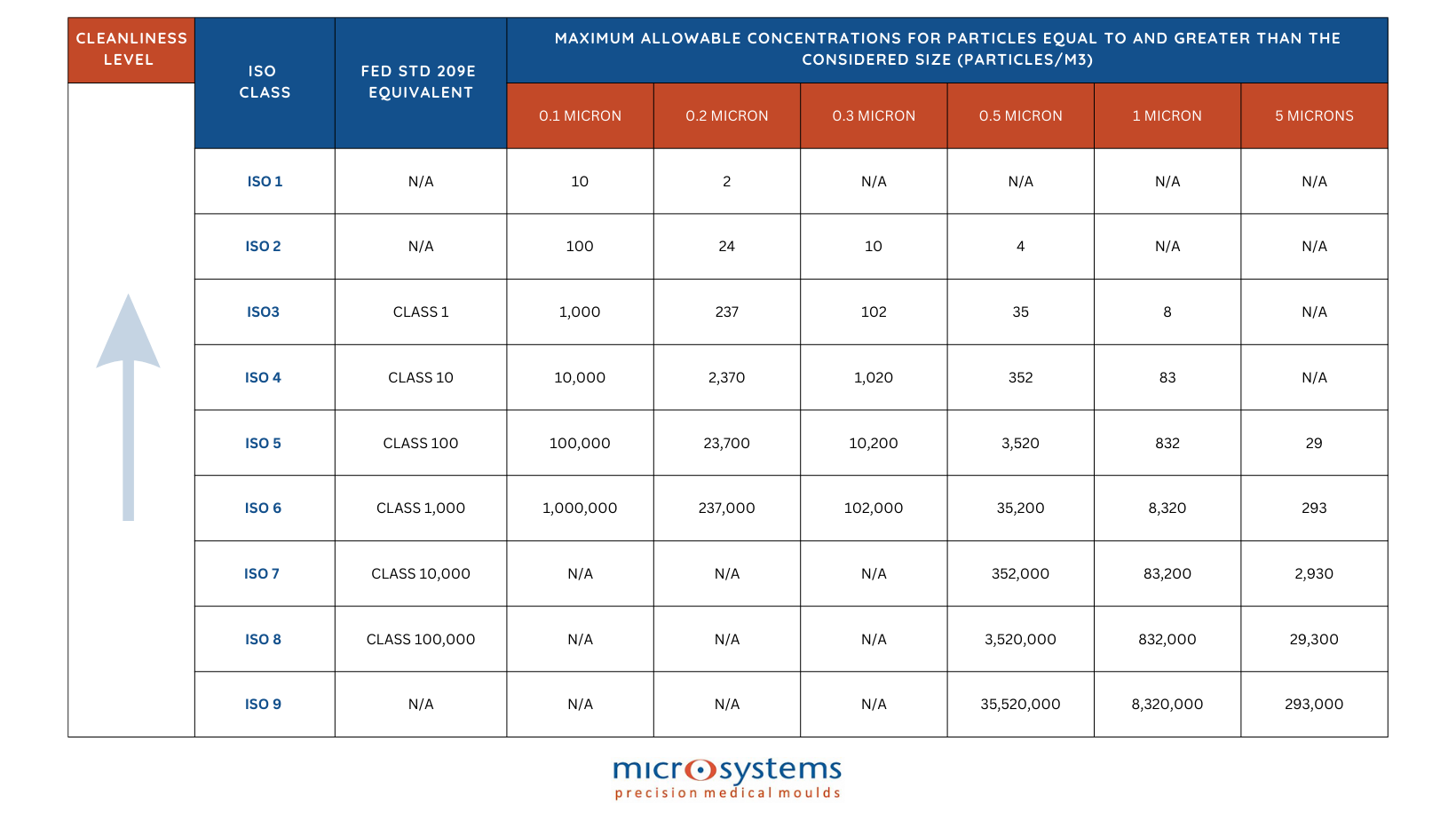
The size of the particles that will need to be filtered out is one of the most crucial factors to comprehend when constructing a cleanroom. Since an environment must always contain no more than 0.23 particles at 0.1 micron/m2, ISO 1 cleanrooms are extremely uncommon in practice, as only a handful of locations worldwide are capable of creating this kind of tightly controlled environment.
In an ISO 7 cleanroom, for instance, the density of particles smaller than 5 microns must be fewer than 2,930/m3. How small is a micron? You can think that a human hair has a diameter of about 100 microns, and the human eye can only perceive particles that are at least 50 microns in size. That’s how small a micron is!
Generally, the degree of cleanliness needed in the room depends on the work that will be done there, as the more prone a product is to contamination, the higher the quality of cleanliness needed. The room hence must be kept in good condition to adhere to the requirements for: Cleanliness, Temperature, Humidity, Pressure, Air changes per hour and Flow rate.

What ISO Cleanroom Classifications for Medical Devices?
Manufacturing of medical devices normally takes place in cleanrooms with an ISO classification of 5 (Class 100) to 8 (Class 100,000). Packaging for medical devices is normally carried out in ISO 7 (Class 10,000) Cleanrooms or ISO 8 (Class 100,000) cleanrooms, together with an ISO 8 (Class 100,000) gowning room.
ISO 7 Cleanroom, in comparison to other classifications, is less expensive to build and uses less energy since it requires fewer air changes. The room requires airlock at any entry point, an internal air pressure between +25 and +30 pascal, and 30 – 60 air changes every hour. Numerous building materials, such as hard walls, monobloc, PVC, glass, etc., can be used to create an ISO 7 Cleanroom, as they are smooth, resistant to common detergents, and simple to clean.
Do’s and Don’ts inside Cleanroom
A thorough risk analysis is necessary to maintain the integrity of a cleanroom, and the suggestions made to reduce those risks are often written in the cleanroom’s Standard Operating Procedures (SOP). In order to keep the needed high standards of cleanliness for cleanrooms, all users must undergo frequent thorough training on these SOPs, and only authorised persons should be allowed to enter the cleanrooms.
Do’s:
- Leave all personal items outside of the cleanroom before entering the cleanroom.
- Wear proper cleanroom gowning materials and protection kit before entering the cleanroom.
- Practice self-control to avoid scratching, touching body parts, etc. Practice good personal hygiene.
- Clean all surfaces regularly with dedicated cleaning equipment to remove contamination.
- Always keep all doors closed to maintain the required pressure and environment cleanliness.
- Avoid running or moving quickly than necessary in the cleanroom.
Don’ts:
- Do not eat, drink or smoke in the cleanroom.
- Do not enter the cleanroom sick or feeling unwell.
- Do not wear heavy makeup, perfume, etc. inside the cleanroom.
- Do not turn off any fan filter units and other cleaning equipment.
Why choose medical device manufacturers with ISO Cleanrooms?
As the healthcare sector is renowned for its strict laws and high standards, which guarantee the security of its delicate products,medical device manufacturers need to comply with all applicable laws and regulations on a global scale.
By choosing medical device and part manufacturers with the appropriate ISO Cleanrooms, OEMs could ensure their suppliers meet their high standards for the end products, protecting the end-users, securing medical device certifications and guaranteeing reliability in their production chain. Medical device manufacturers with ISO Cleanrooms like Micro Systems offer their customers a high degree of operational and functional control, and advantages of efficiency, competency, accountability, and ability to adhere to sterility and global regulatory norms. At Micro Systems, a complete process from mold design through micro mold manufacturing and development to validation are carried out within an ISO Class 7 cleanroom, and backed up by an extensively equipped Metrology department to ensure the highest quality and assurance.

Micro Systems specializes in the design, manufacture and validation of ultra precision micro molds for the medical, pharmaceutical and optical markets, at the same time, the development and use of micro and nano technologies in the design and manufacture of injection molded components. We have a dedicated micro molding facility, and have ISO13485 and ISO9001 certifications, and an ISO Class 7 Cleanroom. For more information, please Contact us or visit our website.





